 Trent R. Northen, Gary Siuzdak et al
Trent R. Northen, Gary Siuzdak et al
Nature 2007, 449, pp. 1033-1036
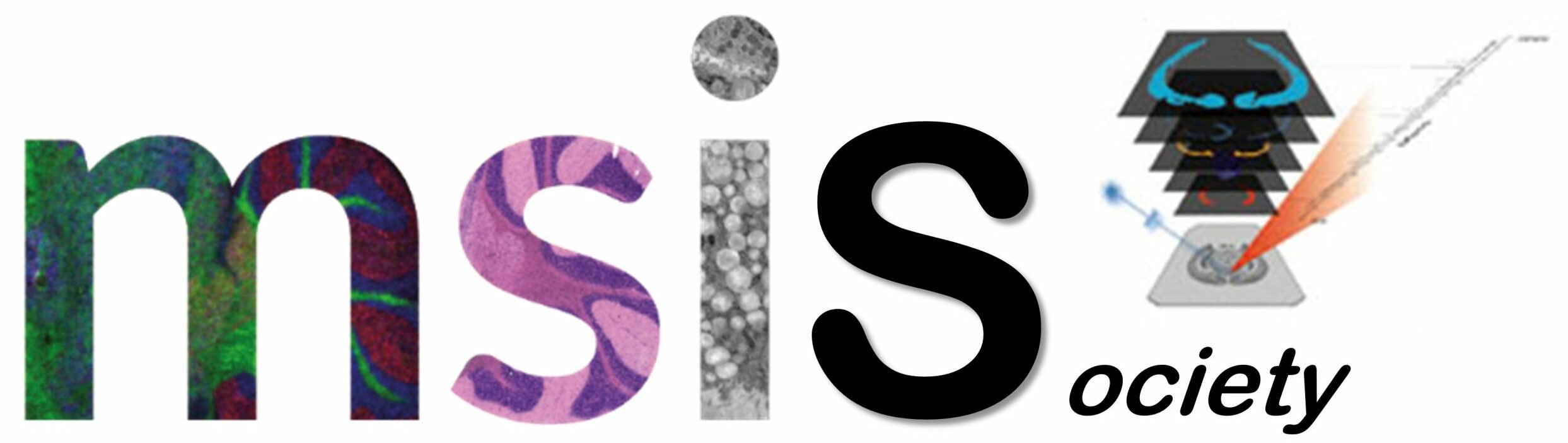
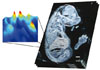
The ability of mass spectrometry to generate intact biomolecular ions efficiently in the gas phase has led to its widespread application in metabolomics, proteomics, biological imaging, biomarker discovery and clinical assays (namely neonatal screens). Matrix-assisted laser desorption/ionization (MALDI) and electrospray ionization have been at the forefront of these developments. However, matrix application complicates the use of MALDI for cellular, tissue, biofluid and microarray analysis and can limit the spatial resolution because of the matrix crystal size (typically more than 10 um), sensitivity and detection of small compounds (less than 500 Da). Secondary-ion mass spectrometry has extremely high lateral resolution (100 nm) and has found biological applications, although the energetic desorption/ionization is a limitation owing to molecular fragmentation. Here we introduce nanostructure-initiator mass spectrometry (NIMS), a tool for spatially defined mass analysis. NIMS uses 'initiator' molecules trapped in nanostructured surfaces or 'clathrates' to release and ionize intact molecules adsorbed on the surface. This surface responds to both ion and laser irradiation. The lateral resolution (ion-NIMS about 150 nm), sensitivity, matrix-free and reduced fragmentation of NIMS allows direct characterization of peptide microarrays, direct mass analysis of single cells, tissue imaging, and direct characterization of blood and urine.
No responses yet