 Mohammadreza Shariatgorji, Anna Nilsson, Patrik Källback, Oskar Karlsson, Xiaoqun Zhang, Per Svenningsson and Per E. Andren
Mohammadreza Shariatgorji, Anna Nilsson, Patrik Källback, Oskar Karlsson, Xiaoqun Zhang, Per Svenningsson and Per E. Andren
J Am Soc Mass Spectrom. 2015 Jun;26(6):934-9. Epub 2015 Mar 28.
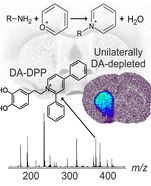
Many neuroactive substances, including endogenous biomolecules, environmental compounds, and pharmaceuticals possess primary amine functional groups. Among these are catecholamine neurotransmitters (e.g., dopamine), many substituted phenethylamines (e.g., amphetamine), as well as amino acids and neuropeptides. In most cases, mass spectrometric (ESI and MALDI) analyses of trace amounts of such compounds are challenging because of their poor ionization properties. We present a method for chemical derivatization of primary amines by reaction with pyrylium salts that facilitates their detection by MALDI-MS and enables the imaging of primary amines in brain tissue sections. A screen of pyrylium salts revealed that the 2,4-diphenyl-pyranylium ion efficiently derivatizes primary amines and can be used as a reactive MALDI-MS matrix that induces both derivatization and desorption. MALDI-MS imaging with such matrix was used to map the localization of dopamine and amphetamine in brain tissue sections and to quantitatively map the distribution of the neurotoxin β-N-methylamino-L-alanine.
No responses yet